When it comes to non-resorbable membranes, many clinicians remain confused by the 3 different types of non-resorbable membranes. In this post, we will quickly review the different types of PTFE membranes, explain why microporous ePTFE membranes (MP-ePTFE), such as Cytoflex Tefguard are probably the best current option, and review an interesting case that examined the clinical, radiological, and histological outcomes of guided-bone regeneration using the novel microporous expanded polytetrafluoroethylene membranes (MP-PTFE membranes).
-
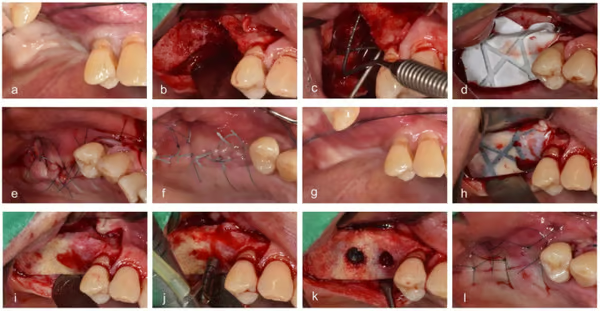
- Surgical procedures. (a) Before vertical ridge augmentation, (b) flap reflection for augmentation, (c) measuring the defect, (d) grafting bone material and membrane, (e) suturing, (f) healing state when visiting for removal of stitches, (g) before reentry, (h) flap reflection for implant placement, (i) removing the membrane, (j) drilling, (k) implant placement, and (l) suturing.
-
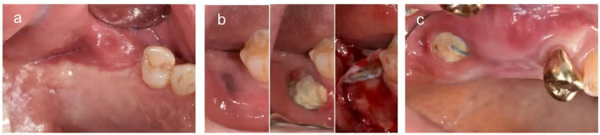
- Representative cases. (a) No exposure, (b) exposure with membrane removal during the healing period, and (c) exposure without membrane removal until implant placement.
-
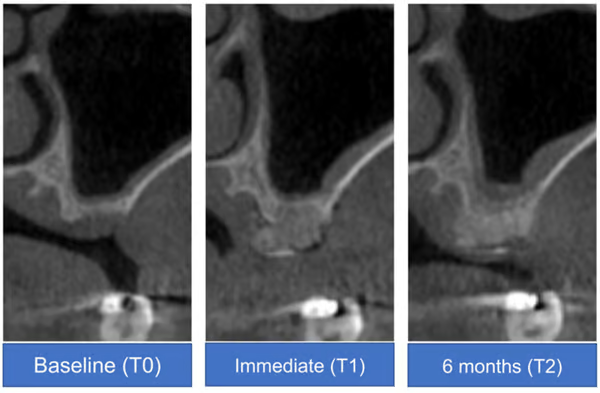
- Cone-beam computed tomography analysis of a representative case at baseline (T0), immediately after surgery (T1), and at 6 months after surgery (T2)
-
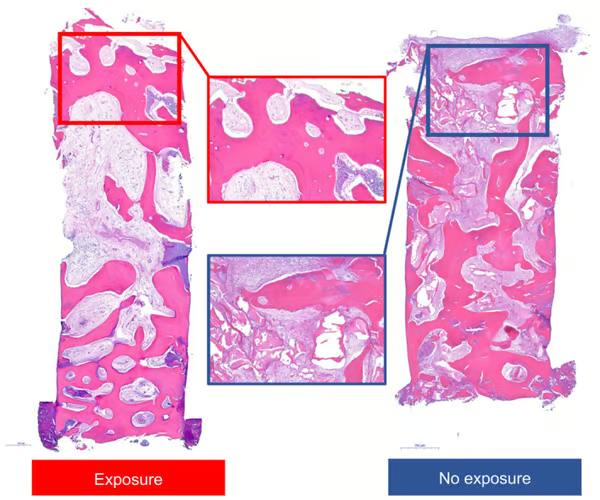
- Histologic views of representative specimens with or without exposure. Images represent entire and high-magnification views.
Source: Ji JG, Yu JA, Choi SH, Lee DW. Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up. Materials (Basel). 2021 Jul 8;14(14):3828. doi: 10.3390/ma14143828. PMID: 34300744; PMCID: PMC8307707.
The Short History of PTFE Membranes and the Next-Generation Microporous ePTFE Membranes
Non-absorbable PTFE membranes were developed because, despite the need for a 2nd surgery (they are non-absorbable), in certain clinical scenarios they can be preferable over absorbable collagen membranes, especially when maintaining the integrity of the regeneration site is a significant priority and you need a more effective barrier material.
The original PTFE membranes, popularized by the GoreTex brand, were called expanded polytetrafluoroethylene membranes, or ePTFE for short. However, these original e-PTFE membranes had some major shortcomings including, a high susceptibility to bacterial contamination, and a requirement for primary closure to prevent membrane exposure.
Dense PTFE Membranes, or d-PTFE membranes sold under the brand name Cytoplast, were developed to improve upon the original ePTFE membranes. d-PTFE Membranes were manufactured with much smaller pore sizes (<0.3 µm), which effectively blocked all bacterial infiltration. The dense structure of d-PTFE membranes helps in reducing the risk of bacterial contamination and infection, which is crucial for maintaining the soft-tissue anatomy and ensuring successful bone regeneration. Furthermore, because of the improved nature of the material d-PTFE membranes do not require primary soft tissue coverage, which simplifies the surgical procedure and reduces the need for extensive flap manipulation. Finally, many clinical studies have shown that exposure of d-PTFE membranes has less negative impact on clinical results compared to e-PTFE membranes. This means that even if the membrane becomes exposed, the clinical outcomes are not significantly compromised.
Despite the many significant improvements offered by d-PTFE membranes over the original e-PTFE membranes, the d-PTFE membranes still had some issues. Specifically, because of their underlying structure these membranes have poor tissue attachment and there is a tendency for slipping and exposure of the graft material when a dPTFE membrane is used during bone augmentation procedures. Furthermore, while the tiny pore size of the dPTFE membranes block bacterial infiltration into the site, at the same time the non-porous nature of the barrier limits nutrient permeation across the membrane, which effects the ultimate healing of the site.
To address these issues with dPTFE membranes, microporous PTFE membranes, (MP-ePTFE), such as Cytoflex Tefguard, were developed. The micro-pores on the MP-ePTFE membranes are miniscule enough to block bacterial penetration keeping the site safe from infection, but the micro-porous material still allows nutrient permeation across the membrane enhancing healing. As such the MP-ePTFE membranes, like the d-PTFE, do not require primary closure. Furthermore, the texture of these materials enhances soft tissue attachment which prevents early flap sloughing and exposure. The textured Cytoflex Tefguard also has superficial macro texture overlapping the micro pore texture on both surfaces, providing additional grips for flap attachment, and easier suture closure.
Overall, the microporous PTFE membranes, such as Cytoflex Tefguard, provide all the benefits of dPTFE membranes, while also providing better handling and healing properties. As such, they offer the best current option for clinical cases that require a non-resorbable membrane.
Clinical Study with Microporous ePTFE Membranes
A recent study, photos above, examined the use of a titanium-reinforced MP-ePTFE membrane for vertical ridge augmentation before dental implant placement. The study demonstrated that a titanium-reinforced MP-ePTFE membrane can successfully be used for vertical ridge augmentation of severely resorbed ridges in posterior areas with or without exposure. Specifically,
There was a significantly lower area of residual bone graft material in the exposed group, and there was no significant difference in the vertical height change in the buccal side between immediately after augmentation procedure and the time of reentry for implant placement. However, all implants functioned well regardless of the exposure during the observation period. The results of this clinical study suggested that vertical ridge augmentation around implants using titanium-reinforced MP-ePTFE membrane can be successful. Further studies are needed to confirm our findings.